Abstract
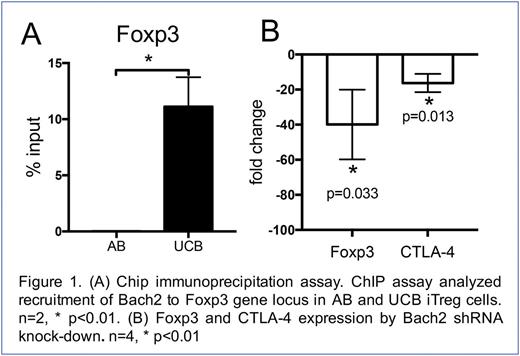
Early clinical experience identifies third party allogeneic natural T regulatory cells (nTreg) to be safe and effective in acute GVHD (aGVHD) prophylaxis (CG Brunstein et al. 2016). However the low proliferative potential of nTreg remains a challenge to broader clinical application. Inducible Treg (iTreg) can reestablish tolerance in settings where Tregs are decreased or defective. However instability of expression of Transcription factor Fork head box P3 (Foxp3), essential for CD4+ iTreg induction and function, also poses a significant barrier to successful clinical application. Further understanding of Foxp3 transcription factor partners and their function is emerging. Foxp3 is known to regulate Treg function through cooperation with Nuclear Factor of Activated T-Cells (NFAT1). More recently, broad complex-Tramtrack-Bric-a-brac domain (BTB) and Cap'n'collar (CNC) homology 1, basic leucine zipper transcription factor 2 (BACH2) has been shown to repress effector programs to stabilize Treg-mediated immune homeostasis in murine KO studies (Roychoudhuri R. 2013). However, little is known in normal human CD4 T cells what role, if any, BACH2 may play in Foxp3+ regulation. Previous work by this group identified higher BACH2 expression in UCB vs. AB CD4+ T cells, and further that BACH2 regulates expression of IL-2 in UCB CD4+ T-cells lacking normal expression of NFAT1 protein (M. Lesniewski et al. 2008). In our current studies, we compared BACH2 function in Foxp3+ iTreg derived from UCB vs. AB naïve CD4+CD45RA+ cells. We observe that: i.) naïve UCB CD4+ T cells highly express BACH2, 21-fold higher compared to AB CD4+ T cells (including protein and mRNA expression (n=6; p<0.01), ii.) Foxp3+ expression is 2.5-fold higher in iTreg derived from UCB CD4+CD45RA+ T cells vs. AB (86.8 ± 3.13 vs. 34.3 ± 4.2, n=12; p<0.001), iii) absolute number of Foxp3+ iTreg generated from UCB vs. AB CD4+CD45RA+ T cells is 4-fold higher (3.8 x 106 vs. 1.0 x 106), in standard iTreg differentiation conditions, e.g. CD2/3/2/28 stimulation in X-VIVO15 media with 5 ng/ml transforming growth factor 𝝱 (TGF𝝱), iv.) suppressive function of UCB Foxp3+ iTreg is more potent in mixed lymphocyte culture compared to AB Foxp3+ iTreg (89% vs. 58% inhibition of T cell proliferation in 1:1 ratio, n=9; p<0.001), v.) UCB iTreg exhibit higher surface PD-1 and CTLA-4 MFI expression (PD-1: UCB: 2835 vs. AB: 1398; p<0.01, CTLA-4: UCB: 1558 vs. AB: 653; p<0.001, n=9). Loss of BACH2 expression in UCB CD4+ T cells results in 42% loss of Foxp3 expression by FACS (Empty shRNA: 78.8% vs. Bach2 shRNA: 46.2%, n=7; p<0.001) in transient shRNA knock-down experiments. Cross-linking chromatin immunoprecipitation (ChIP) showed BACH2 binding to the Foxp3 promoter in UCB iTreg but not AB iTreg (n=2, *; p<0.01) (Figure 1A). BACH2 inhibition via shRNA knockdown notably results in 36-fold reduction of Foxp3 mRNA expression (n=4, *; p<0.01) and 16-fold reduction of Foxp3 down-stream CTLA-4 (n=4, *; p<0.01) by qPCR (Figure 1B). Collectively, these data support the hypothesis that BACH2 is an important transcription factor partner that serves to stabilize Foxp3+ inducible regulatory T cells derived from UCB CD4+ naïve T cells.
No relevant conflicts of interest to declare.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal